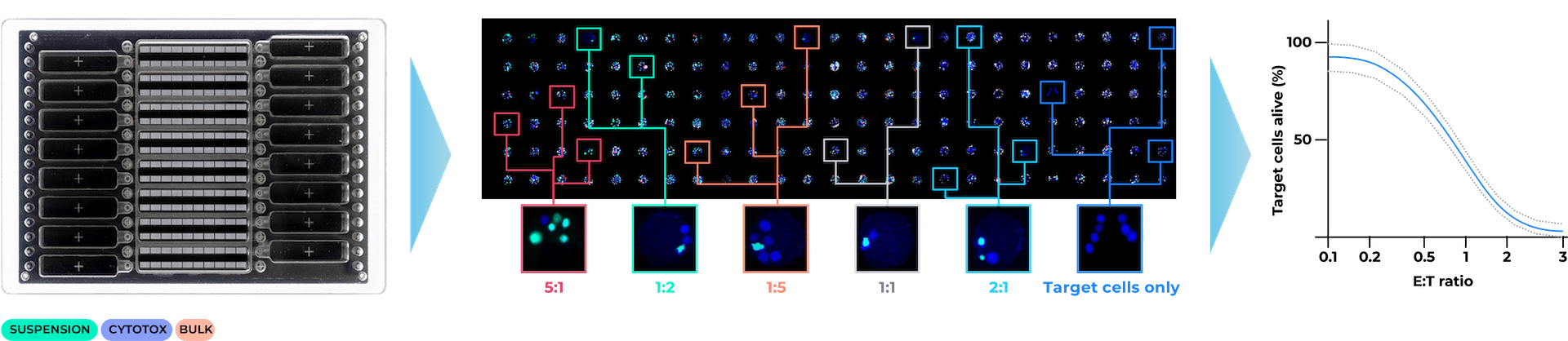
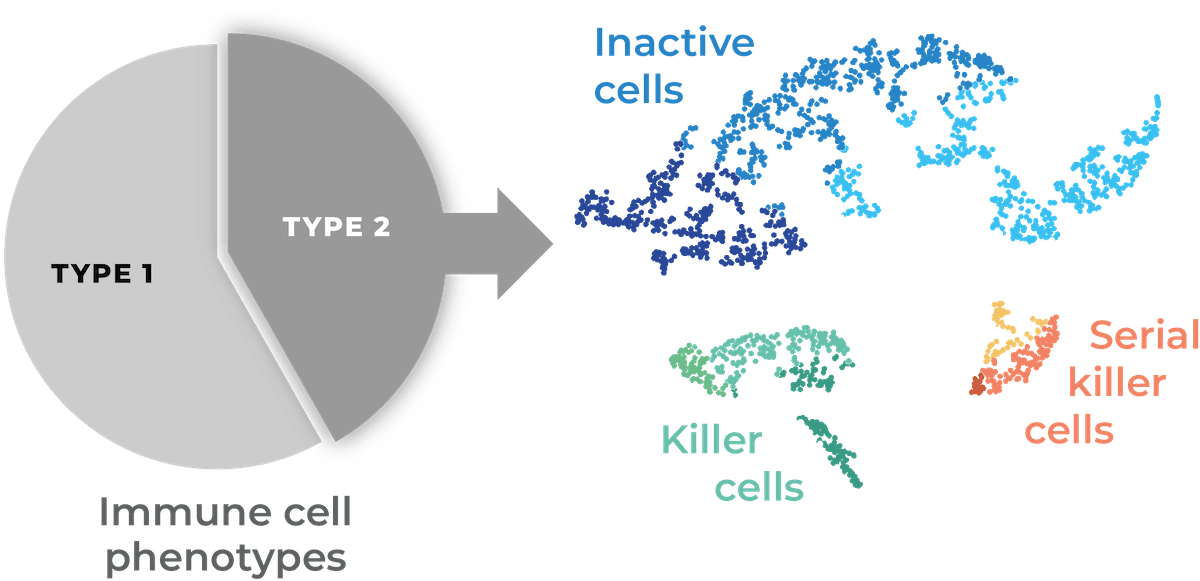
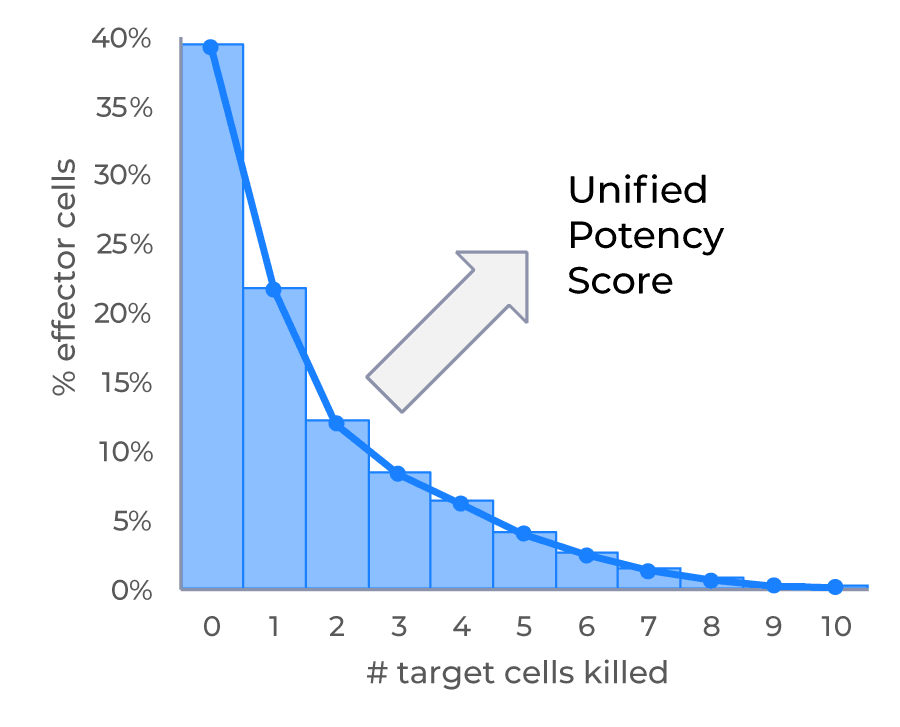
High throughput parallel screening of multiple Effector : Target (E:T) ratios for cell mediated cytotoxicity kinetic analysis using low sample volume
Learn how VivaCyte can generate multiple dose-response analyses in a single CC-Array plate, based on a cell-mediated cytotoxicity assay carried out at multiple E:T ratios